Research outcomes
Unraveling the role of supersulfides in regulating mitochondrial function and longevity
NAIST researchers highlight the crucial role of cysteinyl-tRNA synthetase in producing supersulfides to uphold mitochondrial energy metabolism and preserve protein quality
Supersulfides are gaining prominence for their occurrence as low-molecular-weight thiols or persulfidated cysteine residues, observed more frequently in both prokaryotic and eukaryotic cells. These compounds, which are characterized by sulfur-sulfur bonds, play key roles in energy metabolism, embryonic development, cardiac function, tumorigenesis, innate immunity, antiviral defense, and the prevention of chronic pulmonary disorders. Cysteinyl-tRNA synthetase (CARS) is pivotal across diverse organisms for synthesizing and integrating these supersulfides into proteins. However, the in vivo physiological functions of supersulfides produced by CARS remain unclear.
Now, a study conducted by a research team from Japan has revealed that cysteine persulfide (CysSSH)--a supersulfide synthesized by CARS--regulates cellular longevity in budding yeast. This study, published in Redox Biology , was led by Akira Nishimura from Nara Institute of Science and Technology (NAIST).
To investigate the impact of CARS and supersulfides in yeast, the researchers genetically engineered a mutant strain of Saccharomyces cerevisiae. This strain carried a mutated CARS gene capable of protein synthesis but exhibited decreased production of CysSSH.
Initially, the team demonstrated that the mutant yeast exhibited a markedly reduced lifespan compared to normal (or 'wild-type', WT) yeast. About 50% of mutant cells became nonviable within the first week whereas WT cells remained viable for twice the period. Interestingly, the researchers restored the normal longevity in the mutant strain by externally inducing the production of regular CARS in both the cells' cytosol and mitochondria.
Subsequently, the researchers conducted additional experiments to gain a comprehensive understanding of the effects of the introduced mutation and consequently, the significance of CysSSH and associated supersulfides. Their examination revealed that the CARS-deficient yeast mutant suffered from abnormal mitochondrial energy metabolism, as well as an increased stress response throughout the endoplasmic reticulum. However, supplying the mutant cells with supersulfide donors, like Sodium disulfide (Na2 S2 ) could reverse these detrimental effects. "To the best of our knowledge, this is the first demonstration of CARS- and supersulfide-dependent longevity control, mediated by mitochondrial respiration and the regulation of protein quality," highlights Nishimura.
Notably, this study has important implications not only for yeast, but for countless lifeforms. "Since supersulfide-related lifespan regulation mechanisms are likely to be widely conserved in higher organisms including humans, the intake of supersulfides from supplements and other sources may contribute to the prevention of aging and the extension of a healthy life span," remarks Nishimura. Moreover, abnormal mitochondrial metabolism and endoplasmic reticulum stress are hallmarks of various disorders, such as cardiac diseases, Alzheimer's, Parkinson's, and cancer. Thus, supersulfides may hold tremendous untapped potential in the medical field.
Inspired by the results of their study and with eyes on the future, Nishimura concludes, "Given the promising implications of the present experimental findings and the remarkable potency of supersulfides, continued investigation of supersulfides will surely aid the discovery and development of new drugs for challenging diseases."
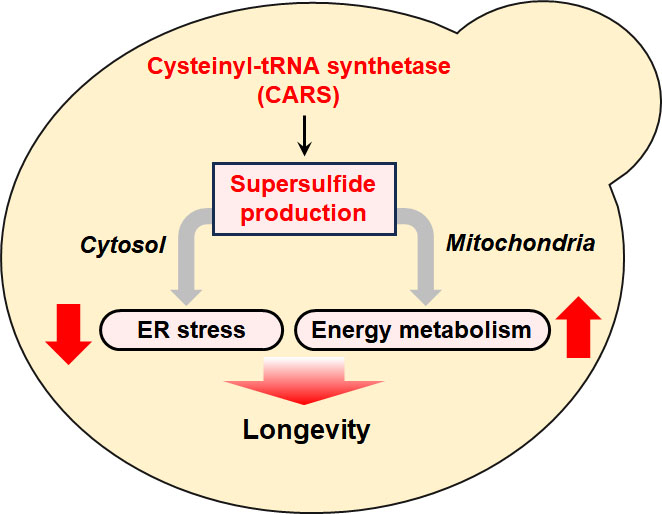
Supersulfides play a fundamental role in longevity: maintaining protein folding in the endoplasmic reticulum (ER) and energy production in mitochondria.
###
Resource
・Title: Longevity control by supersulfide-mediated mitochondrial respiration and regulation of protein quality
・Authors: Akira Nishimura, Sunghyeon Yoon, Tetsuro Matsunaga, Tomoaki Ida, Minkyung Jung, Seiryo Ogata, Masanobu Morita, Jun Yoshitake, Yuka Unno, Uladzimir Barayeu, Tsuyoshi Takata, Hiroshi Takagi, Hozumi Motohashi, Albert van der Vliet, Takaaki Akaike
・Journal: Redox Biology
・DOI:10.1016/j.redox.2023.103018
( January 19, 2024 )
