Research outcomes
A novel mechanism to protect yeast from nitric oxide
A novel mechanism to protect yeast from nitric oxide
Assistant Professor Ryo Nasuno and Professor Hiroshi Takagi (Laboratory of Applied Stress Microbiology, Division of Biological Science, Graduate School of Science and Technology, NAIST) clarified a novel mechanism to protect cells from a toxic compound nitric oxide (NO) in the yeast Saccharomyces cerevisiae.
S. cerevisiae is an important microorganism not only as a model for higher eukaryotes and pathogenic fungi, but also in fermentation industry to produce alcohol beverage, bread, and various useful compounds. NO functions as a signaling molecule involved in a wide variety of biological events, however the excess level of NO induces cell death a cellular dysfunction, called NO stress. Therefore, organisms possess the mechanisms to attenuate NO stress. However, responses of yeast cells to NO stress have not been fully understood.
In this study, they found that fructose-1,6-bisphosphate aldolase Fba1 in glycolytic pathway is inhibited through S-glutathionylation, the disulfide bond between cysteine residue on protein and glutathione, in response to NO stress. Further analysis indicated that the metabolic flow is changed from glycolytic pathway to its bypass, pentose phosphate pathway, in response to NO stress, depending on the S-glutationylation of Fba1. Finally, they demonstrated that the acceleration of NADPH induced by this metabolic switch contributes to NO stress tolerance in yeast (Fig. 1).
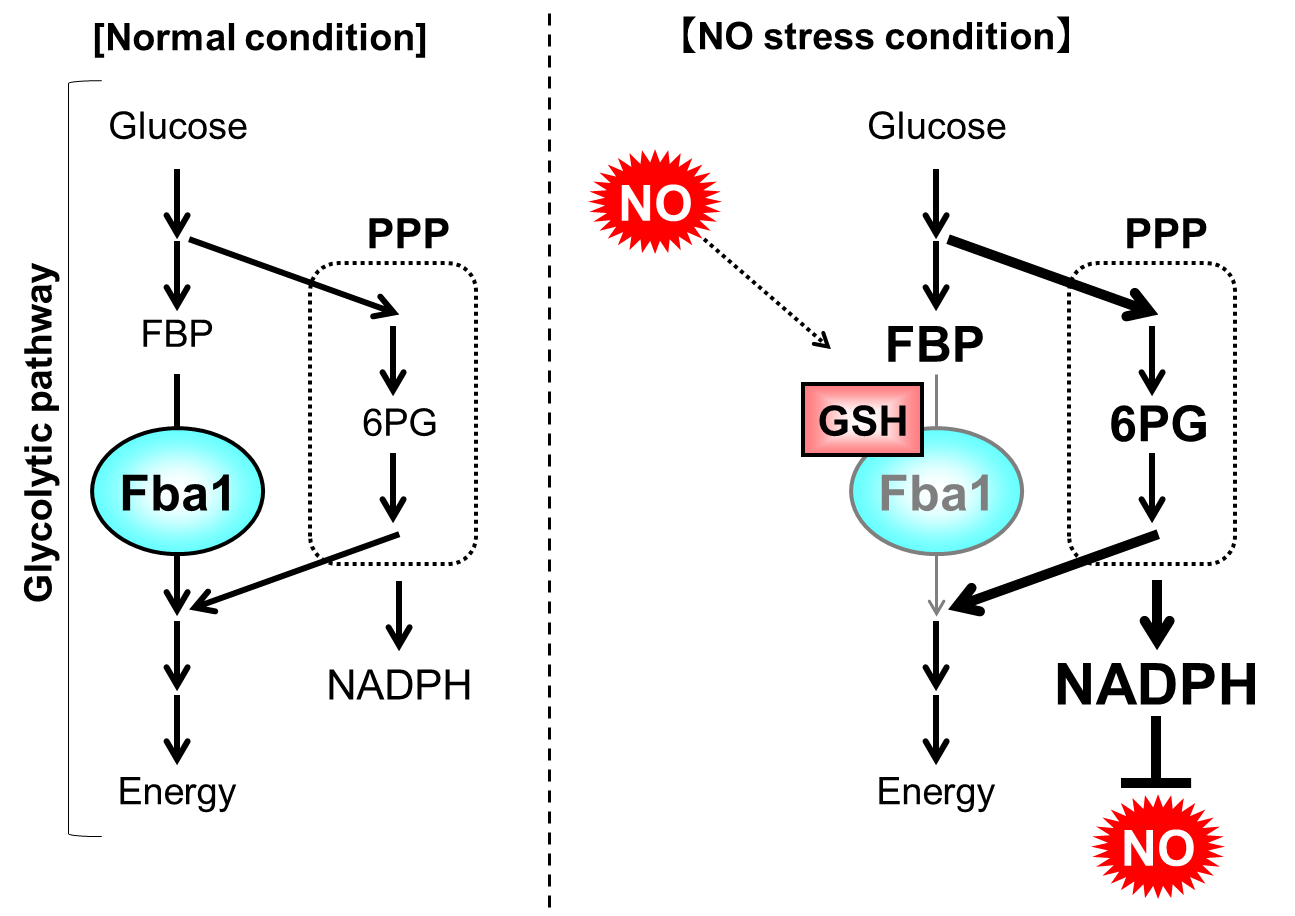
Fig. 1 NO tolerance mechanism mediated by a metabolic switch through the inhibition of Fba1 by S-glutathionylation
When yeast is exposed to NO stress, fructose-1,6-bisphosphate aldolase Fba1 is S-glutathionylated and the its activity is inhibited. Thus, the metabolic flow is switched from glycolytic pathway to PPP, promoting the NADPH synthesis in PPP. Finally, the NO stress tolerance of yeast is upregulated using overproduced NADPH.
Yeast is exposed to NO stress derived from contaminants in fermentation medium and then its fermentation ability is limited (Fig. 2A). On the other hand, the pathogenic fungi are attacked by NO synthesized by the host immune cells during the infection process (Fig. 2B). Thus, the NO tolerance mechanisms in pathogenic fungi are promising targets of novel antifungal drugs. Therefore, the NO tolerance mechanism mediated by S-glutathionylation of Fba1 is an important finding not only in a basic research of NO and yeast, but also to construct the new yeast strains with improved fermentation properties and develop novel antifungals.
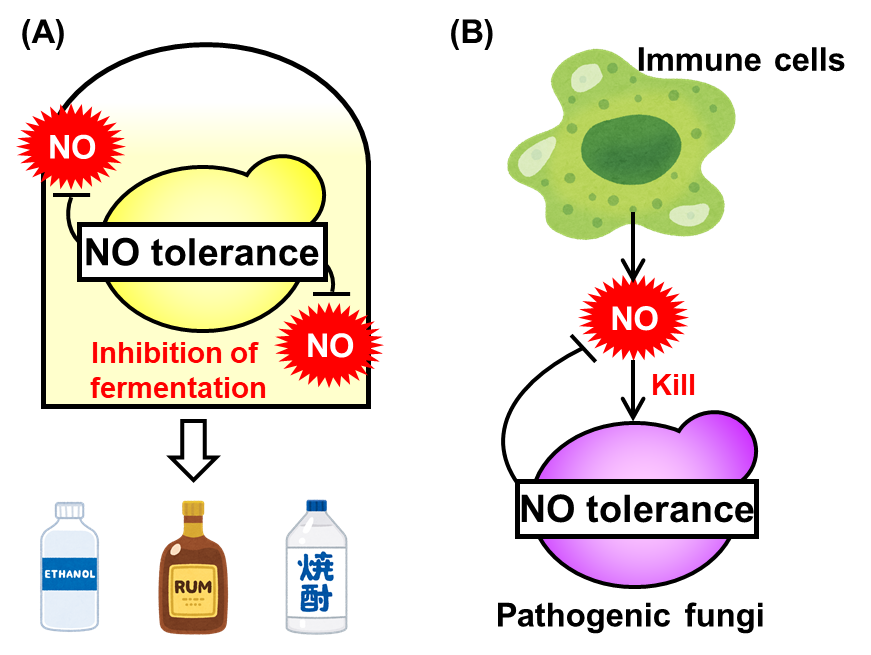
Fig. 2 Relationship of NO tolerance in yeast with (A) fermentation and (B) infection.
(A) During fermentation, yeast is exposed to NO derived from nitrite contained in a medium component molasses and its fermentation ability is limited. (B) Pathogenic fungi, which are closely related species of yeast, are exposed to NO synthesized by immune cell during infection.
Resource
Title: S-glutathionylation of fructose-1,6-bisphosphate aldolase confers nitrosative stress tolerance on yeast cells via a metabolic switch
Authors: Seiya Shino, Ryo Nasuno*, Hiroshi Takagi*
* Corresponding author
Publication: Free Radical Biology and Medicine, DOI: 10.1016/j.freeradbiomed.2022.10.302
Applied Stress Microbiology
https://bsw3.naist.jp/eng/courses/courses305.html
https://bsw3.naist.jp/takagi/?cate=183
( November 10, 2022 )
